
It is much easier to understand what is going on by looking closely at the general case (using "R" groups rather than specific groups) - and then slotting in the various real groups as and when you need to. The reactions between the various sorts of carbonyl compounds and Grignard reagents can look quite complicated, but in fact they all react in the same way - all that changes are the groups attached to the carbon-oxygen double bond. The general reaction between Grignard reagents and carbonyl compounds

If both of the R groups are alkyl groups, the compounds are called ketones. If one (or both) of the R groups are hydrogens, the compounds are called aldehydes. Note: Other carbonyl compounds also react with Grignard reagents, but these are all that are required for UK A level purposes. R and R' can be the same or different, and can be an alkyl group or hydrogen.
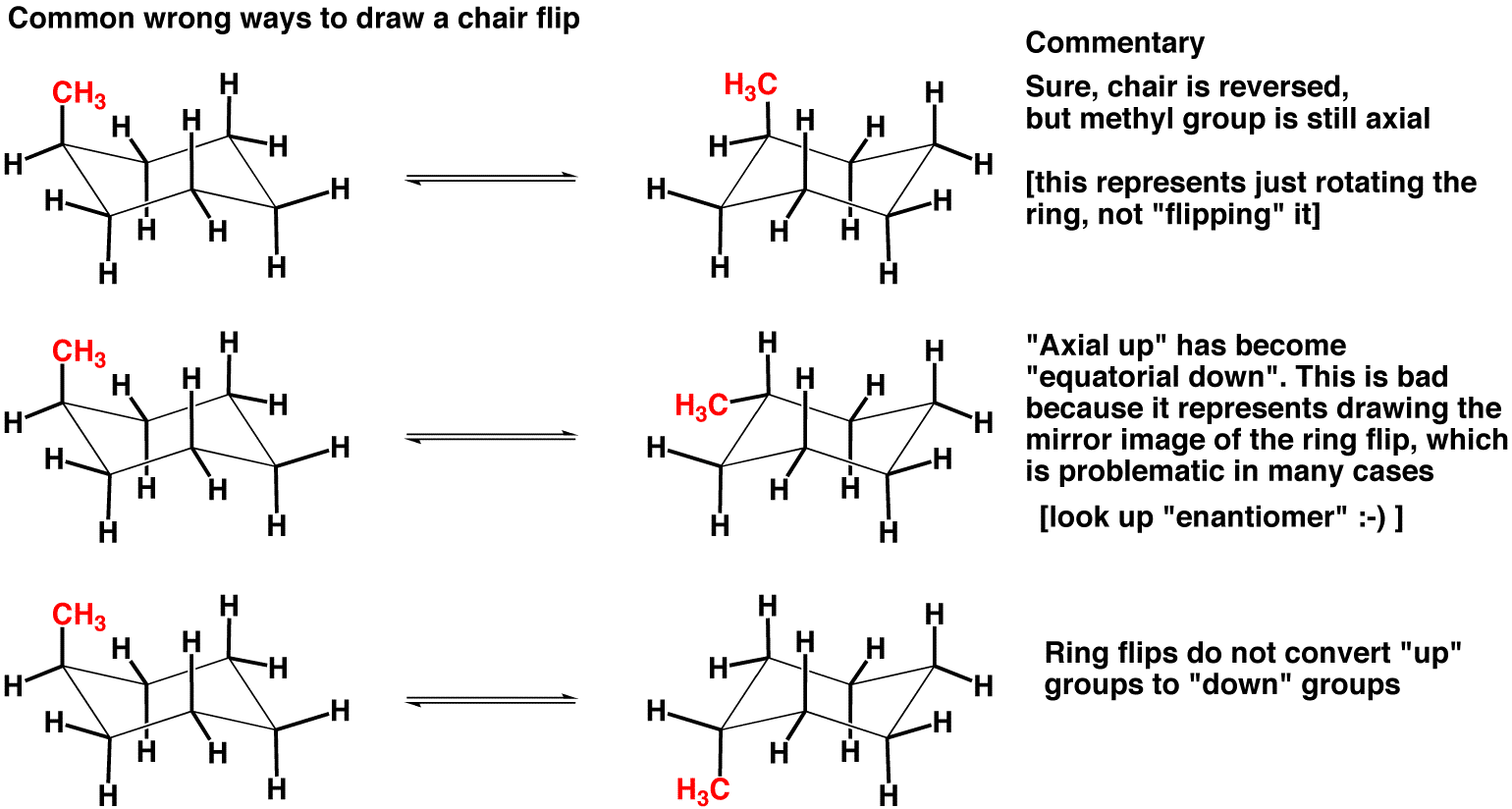
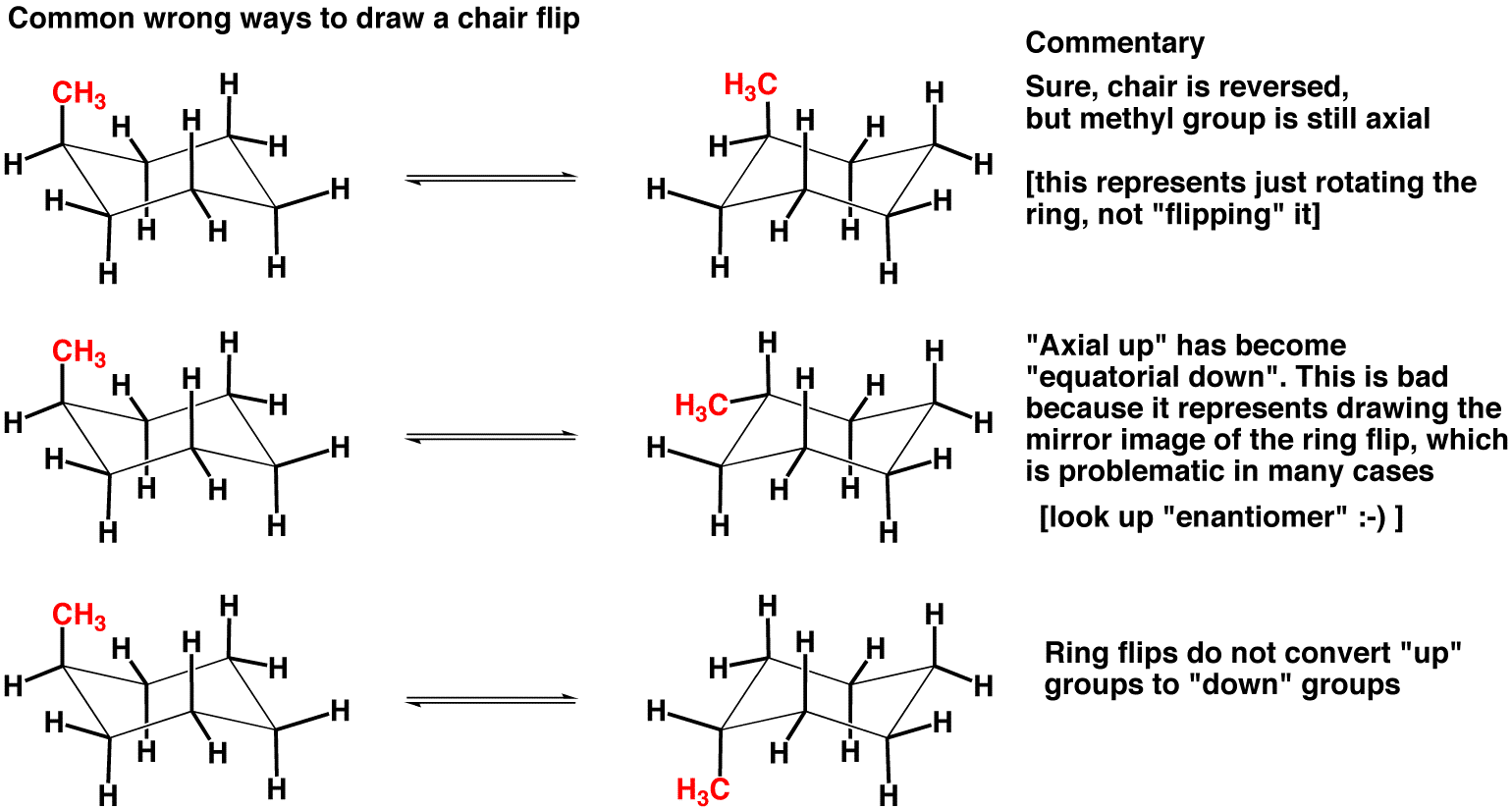
#Cyclohexane in chemdoodle how to
If you are a UK A level student and haven't got copies of these, find out how to get hold of them by going to the syllabuses page to find your Exam Board's web address.Ĭarbonyl compounds contain the C=O double bond. The only way to find that out is to look at old exam papers and mark schemes. Note: What you need to learn about this depends on what your examiners want. What you end up with would be a mixture of ordinary hydrated magnesium ions, halide ions and sulphate or chloride ions - depending on which dilute acid you added. That's actually misleading because these compounds react with dilute acids. The usually quoted equation is (without the red bits):Īlmost all sources quote the formation of a basic halide such as Mg(OH)Br as the other product of the reaction. Typically, you would add dilute sulphuric acid or dilute hydrochloric acid to the solution formed by the reaction with the CO 2.Ī carboxylic acid is produced with one more carbon than the original Grignard reagent. The product is then hydrolysed (reacted with water) in the presence of a dilute acid. In the first, you get an addition of the Grignard reagent to the carbon dioxide.ĭry carbon dioxide is bubbled through a solution of the Grignard reagent in ethoxyethane, made as described above. Grignard reagents react with carbon dioxide in two stages. You can think of it as a sort of half-way stage between magnesium bromide and magnesium hydroxide. The inorganic product, Mg(OH)Br, is referred to as a "basic bromide".
This is the reason that everything has to be very dry during the preparation above. Grignard reagents react with water to produce alkanes. However, by switching to "flammable", I have removed any possible confusion.)Īny reactions using the Grignard reagent are carried out with the mixture produced from this reaction. In fact, confusingly, the two words mean exactly the same thing. (I was challenged by a reader because I had previously used the word "inflammable" rather than "flammable" in this paragraph. Under no circumstances should you try to carry out this reaction without properly qualified guidance. It is a liquid with a boiling point of only 34.5☌, and is extremely flammable. Warning! Ethoxyethane (ether) is very dangerous to work with. The flask is fitted with a reflux condenser, and the mixture is warmed over a water bath for 20 - 30 minutes.Įverything must be perfectly dry because Grignard reagents react with water (see below). Grignard reagents are made by adding the halogenoalkane to small bits of magnesium in a flask containing ethoxyethane (commonly called diethyl ether or just "ether"). For the purposes of this page, we shall take R to be an alkyl group.Ī typical Grignard reagent might be CH 3CH 2MgBr. This page takes an introductory look at how Grignard reagents are made from halogenoalkanes (haloalkanes or alkyl halides), and introduces some of their reactions.Ī Grignard reagent has a formula RMgX where X is a halogen, and R is an alkyl or aryl (based on a benzene ring) group.


 0 kommentar(er)
0 kommentar(er)
